News
- Total Technologies® proposes the PC43K "ALL-IN-ONE" solution!
- Innovative digital solutions for business support in logistics centers, courier companies, and e-commerce
- Quickly scan barcodes with Orbit 7190g barcode reader from Total Technologies®
- Total Technologies® introduces you to the new Honeywell Voice models!
- Total Technologies® offers you two versions of RT10 Tablets, Android and Windows
- From traditional to online commerce through click & collect
- HOW MANAGES DRIVE TYPE RESTAURANTS ''THRU/IN'' SOCIAL DISTANCE
- Remote communication in retail
- HOW PHARMACIES MANAGE SOCIAL DISTANCE
- Clean and Safe!
- HELPING SHAPE THE FUTURE OF YOUR T&L OPERATIONS
- Exceptional durability and reliability for the toughest environments!
- How retailers (groceries) manage social distancing
- Total Technologies® supports social distancing and healthcare with the "Close to me" and "Vita" solution
- Safe and protected with the Voyager 1470g and Xenon 1952g scanners with disinfectant-ready housing
- Total Technologies® and Honeywell are moving, workers health is important “HONEYWELL VOICE WORKS WITH MASKS”
- Did you enroll in the SME program? Total Technologies® can provide you eligible equipments for financing, necessary for your business optimization
- Total Technologies® keeps you up to date with the new products launched “ Granite models with improved performance ”
- Total Technologies®, near you this Black Friday! Time is running, be ready!
- Total Technologies® contributes to the automation of the "self-order" ordering process within McDonald's Romania
- Are you ready to deliver the large volume of Black Friday orders on time? You have very little time left! We offer you the quick and easy solution to implement!
- Total Technologies® continues with discounts!
- Because we think about the future, we also have solutions for your future challenges
- Total Technologies® adds to the portfolio the fixed assets inventory solution by Evidei (assets management)
- A wider range of solutions to your challenges
- Choose to grow your business through efficient methods! Barcode scanning is the road to productivity
- It transforms workforce performance and delivers superior results in automotive
- Digitalisation, the bet of the brave during the pandemic
- The trackers in the barcode jungle
- Total Technologies, FAN Courier & Honeywell Case Studies
- Total Technologies, Gold member ARILOG - Partnership for automation and digitization in logistics
- Maximizing warehouse productivity
- Three ways to prepare your retail warehouse and distribution center teams for the holiday season
- Total Technologies signs partnership with TSC Printronix Auto ID, becoming Silver Partner
- Total Technologies signs partnership with TSC Printronix Auto ID, becoming Silver Partner
- Preparing Your Warehouse for New World Challenges
- 8 years of successful partnership: Total Technologies and BekaertDeslee
- Partnership for the delivery of Christmas joy
- Magic holidays! - Total Technologies team's wishes for partners and collaborators
- Discover the Honeywell solutions that Total Technologies surprises you with at the end of the year
- Total Technologies® and I.O.S. Software Solutions join forces
- Picking by voice - What is it and how it can help you opmitize your logistics?
- Total Technologies® helps you choose a label printer for your business
- We bring technology to romanian business space for over 30 years!
- Universal Robots Solutions
- Operational Intellingence - what is it and how does it helps you to manage your business effectively?
- Total Technologies organizes "How to reduce the execution time and human errors in your warehouse?"
- Why do operations workers prefer mobile terminals over smartphones? - Zebra Enterprise case study
- Why do you need RFID technology? 9 benefits of adopting RFID solutions.
- How do you reduce execution time and human errors in your warehouse?
- Robots won't steal our jobs!
- Electronic Shelf Labels
- 9 advantages that automation can bring to your business
- Technologies that can take your warehouse to the next level
- Total Technologies is organizing the event "Voice demo & coffee"
- Honeywell Voice, Maintenance & Inspection
- #Black Week has started on smartscan.ro
- Voice demo & Coffee - How to address today's workforce challenges in logistics?
- FarEye - the ideal solution for couriers
- Traceability of sterilized medical devices for dental offices
- SOTI - One Platform
- Honeywell 8675i
- Tehnical support and local service
- Honeywell brings Honeywell Voice to Android devices
- Honeywell PX940 – Error-free printer
- Zebra Card Printers
- Evidei - Advanced asset management solution
- Pixevia - Safe, fast and effective shopping!
- The benefits of automation in manufacturing
- A new "TT Coffee & Demo" brand event
- Cubetape - 2 in 1 solution, measuring and scanning
- Bar Tender - Label Management
- NCR self-checkout
- A new version of smartscan.ro at your disposal
- Zebra Mobile Pay Solution
- Total Technologies participated at the sixth edition of "Retail Tech" conference
- In June you have 60% off for PM 43, only on SmartScan.ro
- Total Technologies helps companies become sustainable
- Forging a Sustainable Future: The Power of Partnership
- BarTender Cloud
- AROS - ASBIS Robotic Solutions (AROS)
- Get ready for Black Friday sales boom
- Total Technologies enters the healthcare market, in partnership with Advantech
- Total Technologies participated in the ESG – Positive Business conference
- Total Technologies at EXPO RETAIL 2023: Leading innovations in retail
- Mastering Automation: Key Strategies for Doing More with Less
- Quicktron Robotics Strengthens European Presence Through Strategic Collaboration with Total Technologies
- Human-robot collaboration, the winning synergy in 2024
- Future-oriented: Total Technologies and EP Equipment together, grow smart businesses
- RFID is the future
- Total Technologies joins the vision of SARMIS Capital and Smart ID.
- Merger project between Total Technologies S.R.L. and SMART ID Dynamics S.A.
- Total Technologies® present at the Expo Shop Romexpo, October 9-11!
- Our webshop is always open
- Top of the line DC worker productivity
- #TotalTechnologies & #Honeywell prezent la GoTech Romexpo, 2-3 Octombrie!
- Migrating from Windows to Android with Mobility Edge by Honeywell!
- Total Technologies® proposes the Autonomous Mobile Fetch HMIShelf Robot!
- Honeywell PX940 Industrial Printer by Total Technologies®!
- Total Technologies® invited you to Les RENCONTRES FRANCOPHONES d 'AFFAIRES together with the partners Honeywell and Fetch Robotics!
- Total Technologies® automates your warehouse with Fetch Robots!
- Total Technologies® attended the annual meeting of Vocollect partners from Europe
- Total Technologies® has won the innovation award in SUPPLY-CHAIN 2019!
- Total Technologies® proposes the Honeywell Dolphin CT60 terminal!
- Total Technologies® present at Expo Transit with partners Honeywell and Fetch Robotics!
- Total Technologies® present at Arilog Conference- Keep Your Business CONNECTED To The Future!
- Total Technologies® attends the Honeywell EE&M Partner Conference!
- Total Technologies® introduces you to the new Honeywell CK65!
- Total Technologies® implements mobile autonomous robots in Romania and Moldova!
- Total Technologies® present at Courier & Postal Services Forum- Romania 2019
- Total Technologies® Official Partner Fetch Robotics - Autonomous robots in distribution centers!
- Less than a month until February 9, 2019! Delegated Regulation (EU) 2016/161 for the serialization of medicines!
- The new ScanPal EDA51
- Thor VM1A - The new mobile computer!
- Total Technologies® - present at I&M World - Smart Business Equipment and Solutions!
- Optimize Your Workflow Performance
- The Innovation Award
- Total Technologies® has finalized the implementation of the solution that has led to triple the productivity and increase the accuracy within FAN COURIER warehouses
Less than a month until February 9, 2019! Delegated Regulation (EU) 2016/161 for the serialization of medicines!
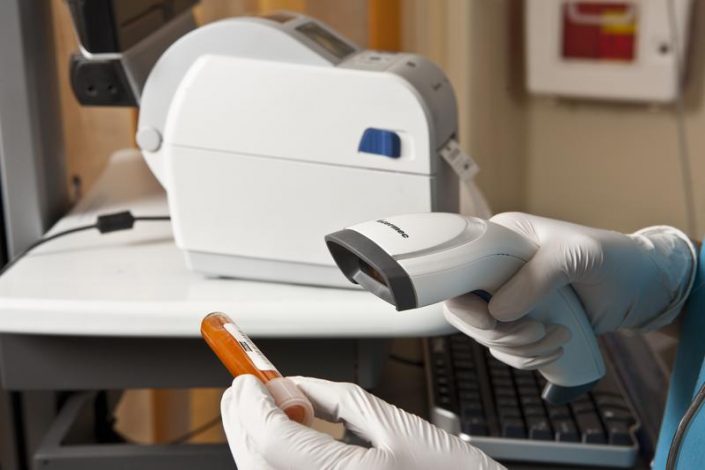
Following Directive 2011 / 62UE and Delegated Regulation 2016/161 establishing a set of detailed rules for the safety elements appearing on the packaging of medicinal products for human use, the latter will have a unique identification code and a device for identification protection against unlawful changes.
This decision was made following the alarming increases of falsification of medicines, so it was decided to eliminate even the fractions from the boxes of medicines, a practice that was only allowed in Romania.
As of February 9, 2019, only prescription drugs that carry the new safety features to be tracked and verified throughout the production and sales chain (Track & Traceability) will be released.
After February 9, 2019, boxes of medicines that do not have the safety elements required by Delegated Regulation (EU) 2016/161 will NOT be able to be included in the circuit.
The autoID digital solutions proposed by Total Technologies® for drug testing to support all traders in the pharmaceutical industry are: ScanPal EDA51 and Voyager 1450g
These offer the possibility of scanning both 2D and 1D barcodes, being a low-cost investment.
See news
- Total Technologies® proposes the PC43K "ALL-IN-ONE" solution!
- Innovative digital solutions for business support in logistics centers, courier companies, and e-commerce
- Quickly scan barcodes with Orbit 7190g barcode reader from Total Technologies®
- Total Technologies® introduces you to the new Honeywell Voice models!
- Total Technologies® offers you two versions of RT10 Tablets, Android and Windows
- From traditional to online commerce through click & collect
- HOW MANAGES DRIVE TYPE RESTAURANTS ''THRU/IN'' SOCIAL DISTANCE
- Remote communication in retail
- HOW PHARMACIES MANAGE SOCIAL DISTANCE
- Clean and Safe!
- HELPING SHAPE THE FUTURE OF YOUR T&L OPERATIONS
- Exceptional durability and reliability for the toughest environments!
- How retailers (groceries) manage social distancing
- Total Technologies® supports social distancing and healthcare with the "Close to me" and "Vita" solution
- Safe and protected with the Voyager 1470g and Xenon 1952g scanners with disinfectant-ready housing
- Total Technologies® and Honeywell are moving, workers health is important “HONEYWELL VOICE WORKS WITH MASKS”
- Did you enroll in the SME program? Total Technologies® can provide you eligible equipments for financing, necessary for your business optimization
- Total Technologies® keeps you up to date with the new products launched “ Granite models with improved performance ”
- Total Technologies®, near you this Black Friday! Time is running, be ready!
- Total Technologies® contributes to the automation of the "self-order" ordering process within McDonald's Romania
- Are you ready to deliver the large volume of Black Friday orders on time? You have very little time left! We offer you the quick and easy solution to implement!
- Total Technologies® continues with discounts!
- Because we think about the future, we also have solutions for your future challenges
- Total Technologies® adds to the portfolio the fixed assets inventory solution by Evidei (assets management)
- A wider range of solutions to your challenges
- Choose to grow your business through efficient methods! Barcode scanning is the road to productivity
- It transforms workforce performance and delivers superior results in automotive
- Digitalisation, the bet of the brave during the pandemic
- The trackers in the barcode jungle
- Total Technologies, FAN Courier & Honeywell Case Studies
- Total Technologies, Gold member ARILOG - Partnership for automation and digitization in logistics
- Maximizing warehouse productivity
- Three ways to prepare your retail warehouse and distribution center teams for the holiday season
- Total Technologies signs partnership with TSC Printronix Auto ID, becoming Silver Partner
- Total Technologies signs partnership with TSC Printronix Auto ID, becoming Silver Partner
- Preparing Your Warehouse for New World Challenges
- 8 years of successful partnership: Total Technologies and BekaertDeslee
- Partnership for the delivery of Christmas joy
- Magic holidays! - Total Technologies team's wishes for partners and collaborators
- Discover the Honeywell solutions that Total Technologies surprises you with at the end of the year
- Total Technologies® and I.O.S. Software Solutions join forces
- Picking by voice - What is it and how it can help you opmitize your logistics?
- Total Technologies® helps you choose a label printer for your business
- We bring technology to romanian business space for over 30 years!
- Universal Robots Solutions
- Operational Intellingence - what is it and how does it helps you to manage your business effectively?
- Total Technologies organizes "How to reduce the execution time and human errors in your warehouse?"
- Why do operations workers prefer mobile terminals over smartphones? - Zebra Enterprise case study
- Why do you need RFID technology? 9 benefits of adopting RFID solutions.
- How do you reduce execution time and human errors in your warehouse?
- Robots won't steal our jobs!
- Electronic Shelf Labels
- 9 advantages that automation can bring to your business
- Technologies that can take your warehouse to the next level
- Total Technologies is organizing the event "Voice demo & coffee"
- Honeywell Voice, Maintenance & Inspection
- #Black Week has started on smartscan.ro
- Voice demo & Coffee - How to address today's workforce challenges in logistics?
- FarEye - the ideal solution for couriers
- Traceability of sterilized medical devices for dental offices
- SOTI - One Platform
- Honeywell 8675i
- Tehnical support and local service
- Honeywell brings Honeywell Voice to Android devices
- Honeywell PX940 – Error-free printer
- Zebra Card Printers
- Evidei - Advanced asset management solution
- Pixevia - Safe, fast and effective shopping!
- The benefits of automation in manufacturing
- A new "TT Coffee & Demo" brand event
- Cubetape - 2 in 1 solution, measuring and scanning
- Bar Tender - Label Management
- NCR self-checkout
- A new version of smartscan.ro at your disposal
- Zebra Mobile Pay Solution
- Total Technologies participated at the sixth edition of "Retail Tech" conference
- In June you have 60% off for PM 43, only on SmartScan.ro
- Total Technologies helps companies become sustainable
- Forging a Sustainable Future: The Power of Partnership
- BarTender Cloud
- AROS - ASBIS Robotic Solutions (AROS)
- Get ready for Black Friday sales boom
- Total Technologies enters the healthcare market, in partnership with Advantech
- Total Technologies participated in the ESG – Positive Business conference
- Total Technologies at EXPO RETAIL 2023: Leading innovations in retail
- Mastering Automation: Key Strategies for Doing More with Less
- Quicktron Robotics Strengthens European Presence Through Strategic Collaboration with Total Technologies
- Human-robot collaboration, the winning synergy in 2024
- Future-oriented: Total Technologies and EP Equipment together, grow smart businesses
- RFID is the future
- Total Technologies joins the vision of SARMIS Capital and Smart ID.
- Merger project between Total Technologies S.R.L. and SMART ID Dynamics S.A.
- Total Technologies® present at the Expo Shop Romexpo, October 9-11!
- Our webshop is always open
- Top of the line DC worker productivity
- #TotalTechnologies & #Honeywell prezent la GoTech Romexpo, 2-3 Octombrie!
- Migrating from Windows to Android with Mobility Edge by Honeywell!
- Total Technologies® proposes the Autonomous Mobile Fetch HMIShelf Robot!
- Honeywell PX940 Industrial Printer by Total Technologies®!
- Total Technologies® invited you to Les RENCONTRES FRANCOPHONES d 'AFFAIRES together with the partners Honeywell and Fetch Robotics!
- Total Technologies® automates your warehouse with Fetch Robots!
- Total Technologies® attended the annual meeting of Vocollect partners from Europe
- Total Technologies® has won the innovation award in SUPPLY-CHAIN 2019!
- Total Technologies® proposes the Honeywell Dolphin CT60 terminal!
- Total Technologies® present at Expo Transit with partners Honeywell and Fetch Robotics!
- Total Technologies® present at Arilog Conference- Keep Your Business CONNECTED To The Future!
- Total Technologies® attends the Honeywell EE&M Partner Conference!
- Total Technologies® introduces you to the new Honeywell CK65!
- Total Technologies® implements mobile autonomous robots in Romania and Moldova!
- Total Technologies® present at Courier & Postal Services Forum- Romania 2019
- Total Technologies® Official Partner Fetch Robotics - Autonomous robots in distribution centers!
- Less than a month until February 9, 2019! Delegated Regulation (EU) 2016/161 for the serialization of medicines!
- The new ScanPal EDA51
- Thor VM1A - The new mobile computer!
- Total Technologies® - present at I&M World - Smart Business Equipment and Solutions!
- Optimize Your Workflow Performance
- The Innovation Award
- Total Technologies® has finalized the implementation of the solution that has led to triple the productivity and increase the accuracy within FAN COURIER warehouses
Terms and conditions
Contact form
Terms and conditions
The company that manages this website (www.smartscan.ro), is SC Total Technologies® SRL, registered with the Trade Register with no. J40 / 15732/1992 and having the Unique Registration Code RO1590813.
Before using the SmartScan online store, we recommend that you read the Terms and Conditions below carefully to make sure you agree with them.
Total Technologies® SRL has the right to change these provisions without prior notification, so we recommend you to check the latest version of the "Terms and Conditions" page in the "Legal" section, which is in the header of the website pages.
Copyright
The entire content of this website: images, texts, graphs, symbols, web graphics elements, scripts, programs and other data is the property of Total Technologies® SRL and its suppliers and is protected by the Copyright Law and the laws on intellectual and industrial property. . The use without the written consent of Total Technologies® SRL of any of the elements listed above is punishable according to the laws in force.
Access to information
Users of the Total Technologies® SRL platform have limited access and only in their personal interest on this website and have no right to download, modify, reproduce, copy, sell / resell or exploit the content in whole or in part, in any other manner, for commercial purposes or contrary to the interests of Total Technologies® SRL, without its prior written consent.
Limitation of liability for products and services
The products and information we offer are transmitted in exactly the same form by our manufacturers, being available within the limit of the stock. Total Technologies® SRL does not offer any warranty, expressly or implicitly, regarding the information, content, materials or products existing in the online store, as well as their suitability for a particular purpose.
Users expressly agree that the use of this online store and the purchase of products or services is at their own risk.
Also, Total Technologies® SRL cannot guarantee the availability in stock of all the presented products and does not assume the responsibility for the incorrect display of the information regarding their existence in stock. The images published on this website are for example, and the products delivered can sometimes differ from the images presented, in any way (color, accessories, appearance, etc.).
Total Technologies® SRL and its suppliers reserve the right to modify the technical specifications of the products without any prior notification. The content of the online store (texts, product descriptions, technical characteristics, images, symbols) is made in close collaboration with the representatives of the respective brands. For this reason, Total Technologies® SRL assumes no responsibility for the inappropriate descriptions of the products presented on this website, these being identical to those made available by the representatives of each brand.
All products marketed through this website are accompanied by the original warranty certificate, issued by the importer or supplier.
The maximum value of the damages that can be paid by Total Technologies® SRL to any client in the case of improper delivery or delivery is the amount of the amount collected Total Technologies® SRL from the respective client.
Regardless of how the order is transmitted (through the online store, by e-mail, telephone or other method), the acceptance of the order, ie the conclusion of the will agreement and the purchase sale contract is made when the customer signs the accompanying tax invoice the product or delivery documents made available by the fast courier company. The tax invoice takes place in the Sale-Purchase Contract. The launch of the order, the automatic email that is sent after receiving the order, the telephone discussions, by email or other methods do not constitute firm acceptance of the order and, as a result, does not mean the conclusion of the distance contract.
Limitation of liability for prices
Total Technologies® SRL reserves the right to modify without first announcing any of the prices of the products, as well as to eliminate some products, to replace them or to introduce new ones. The prices presented include VAT (19%), but not the delivery costs, unless otherwise specifically stated on the online store. The purchase price printed on the invoice will be the same as the one published on the online store at the time of purchase. There is no minimum order price limit.
Limitation of liability for delivery
Total Technologies® SRL reserves the right to contact the clients in order to confirm the orders, before their honor.
The products are delivered anywhere in Romania, within 7 (seven) working days from the moment of confirmation of the order by the TOTAL TECHNOLOGIES SRL staff.
In case the customer is not found at the address mentioned in the order, within the hourly interval agreed with the representative of the courier company, he will return again. If not this time can not make the delivery, the order will be canceled and the product returned to the premises, the customer will bear the costs of a new delivery, regardless of the value of the ordered products.
For orders that exceed the value of 150 lei (without VAT), the delivery of the products is free in the area of coverage of the courier companies. If the locality in which the delivery is to be made is not in the area of coverage of the courier companies, the client will be informed about the transport tax to be paid.
If the value of the order is less than the sum of 150 lei (excluding VAT), the delivery fee falls to the Beneficiary (Buyer).
Total Technologies® SRL reserves the right to delay or cancel deliveries of ordered products if they cannot be honored for independent reasons, such as: fires, explosions, floods, epidemics, strikes, government actions, wars, acts of terrorism, protests, riots, civil unrest or other force majeure impediments, according to Romanian law users.
Limitation of liability for payment
The payment of the ordered products can be made online with bank cards; reimbursement, to the representative of the courier company at the moment of receiving the product or by cash / bank card, when picking up the products from the Total Technologies® SRL showrooms.
Total Technologies® SRL reserves the right to request payment in advance in certain cases, based on a proforma invoice issued and transmitted through the means of communication established in agreement with the client.
Limitation of liability for product warranty
The products purchased through the online store benefit from the warranty provided by the supplier of each brand, in compliance with the legal provisions in force. Thus, each product will be accompanied by the guarantee certificate issued by the manufacturer or importer, which provides, directly or through its representatives, service for that product.
Limitation of liability for user comments
Users who visit Total Technologies® SRL's website may comment on its website and the products it offers, which may be published after the prior approval of the administrator. Total Technologies® SRL reserves the right not to publish comments with illegal, obscene, threatening, defamatory content, which in any way disturbs the private life of other persons, infringes the intellectual property rights, contain viruses, texts regarding various promotional campaigns, letters in chain, mass emails or any other form of spam. Total Technologies® SRL will not be liable nor will it be obliged to pay damages for any form of damage caused by such comments or communications.
In the case of sending materials / documents, other than those related to the process of buying / selling some products, it is considered that the user confers Total Technologies® SRL and its affiliates / associates the non-exclusive, unlimited, free, irrevocable and retransmissible right to use them, reproduce, modify, adapt, publish, translate, as well as the right to distribute them anywhere in the world, by any means.
The user guarantees that he has all the rights to the content he transmits to be published on the website, by any means, so that, by using this content, he does not cause any harm to any person. Total Technologies® SRL assumes no responsibility for the possible damages caused by the use of this information, being offered under the same limitation of liability as the rest of the content.
Limitation of liability for links to other online stores, server, browser
The website www.smartscan.ro is hosted on the servers of a third company. Total Technologies® SRL will not be held liable for any errors that may occur, regardless of the reasons for their occurrence, including changes to the website, settings or updates. Also, Total Technologies® SRL will not be liable for errors arising due to the use of certain navigation programs (browser).
Total Technologies® SRL is not responsible for the content, quality or nature of the Internet pages that can be accessed through links on the smartscan.ro website. For the respective pages, the integral responsibility is borne by their owners.
The right to return the products
The customer has the right to notify in writing Total Technologies® SRL that he gives up the purchase, without penalties and without invoking a specific reason, within 14 working days of receiving the product.
Within this term, the product purchased in the original packaging, with all the accessories and without showing any trace of damage or wear, will be returned, the customer bearing the shipping costs according to the Romanian laws.
This right can not be invoked for products picked up from our premises, for which the sample was tested or installed.
If the product is returned in a state that no longer allows the sale of the product as new, Total Technologies® SRL reserves the right to request compensation for its return to the initial stage (if possible) or to cover the price difference resulting from the sale. of the product as used. If none of these options is accepted by the customer, the product will be shipped back to him at his expense. This clause applies according to O.G. 34/2014, in the case of the purchase of products using the techniques of remote communication, applying the definitions contained in O.G. 34/2014 art. 2 paragraph 7.
Any such request / notification must be sent in writing to totaltech[at]totaltech.ro.
Protection of personal data
Total Technologies® SRL respects the private character and security of the processing of personal data of each person who visits or orders products on this site being registered under Nr. 27939 to the National Supervisory Authority for the Processing of Personal Data. - check if it is also valid for Total Technologies® !!!
According to the requirements of Law no. 677/2001 for the protection of persons regarding the processing of personal data and the free movement of these data, modified and completed, and of Law no. 506/2004 regarding the processing of personal data and the protection of privacy in the electronic communications sector, Total Technologies® SRL will manage, in safe conditions and only for the specified purposes, the personal data provided.
It is obligatory to provide the data, these being necessary to be able to be contacted, to be able to issue the transaction documents and to deliver the order. Refusal to provide this information leads to the impossibility of processing the placed orders.
The registered information is intended for use by Total Technologies® SRL and is communicated (partially) only to the following recipients: the fast courier company that delivers the products, the company that provides the service, in the case of the products that require installation / interventions within the guarantee term and the banking institutions or financial, for card payments (online or directly in the showroom).
If you would like to receive information about the products or services offered by Total Technologies® SRL, please check the subscription box on the newsletter at the end of an order or subscribe directly through the dedicated section of the website. In case you wish to subsequently receive communications from Total Technologies® SRL, please unsubscribe using the link in each newsletter.
According to the Law no. 677/2001 you benefit from the right of access and intervention on the data, as well as the right not to be subjected to an individual decision. At the same time, you have the right to oppose the processing of personal data concerning you and to request their deletion. In order to exercise these rights, you can address a written request sent by email (totaltech[at]totaltech.ro), dated and signed on the address of Total Technologies® SRL, which can be found on the contact page. However, it should be specified that, in accordance with the provisions of the legislation in force, the personal data cannot be erased from the financial, fiscal and management documents issued by Total Technologies® SRL, these processing having a mandatory character, and Law no. 677/2001 provides for the exclusion of the right of opposition in this case. If some of the data provided is incorrect, please inform us as soon as possible at totaltech[at]totaltech.ro.
Security of personal data
Your personal data will be used by Total Technologies® SRL and its suppliers only for the stated purpose of this website, in accordance with the legal provisions.
The information in the order form will be used to send the order confirmation, any promotions, periodic newsletters, etc. Total Technologies® SRL undertakes not to make public or sell databases containing information regarding personal data.
The personal data may be transmitted to the authorities in the right to verify the commercial transactions, only at their justified request.
Information security
The security level of transactions, purchasing sessions and the introduction of personal data is in accordance with the requirements of this industry. This website uses security measures against the loss, alteration or misuse of the information that is under our control by securing the platform with Cross-Site Scripting. However, Total Technologies® SRL assumes no responsibility for the loss of information caused by bugs, "hacking" attacks or errors in the software with which the online store is designed and hosted.
Promotions and contests
Total Technologies® SRL establishes the regulations of the promotions and competitions that it organizes and informs the possible participants through its own online store or through e-mail messages. Promotions benefit only those orders that comply precisely with the rules posted on the site. Also, promotions apply only to orders that are registered during their validity and only within the limit of the available stock.
Total Technologies® SRL does not guarantee the availability on stock of the products for promotion and may interrupt or cancel at any time without any prior notification.
Waste electrical and electronic equipment (WEE)
Waste electrical and electronic equipment (WEEE) may contain hazardous substances that have a negative impact on the environment and human health if not collected separately.
Considering the provisions of GEO 195/2005, regarding the protection of the environment and O.U.G. 5/2015 regarding the waste of electrical and electronic equipment, the buyers have the obligation not to eliminate the waste of electrical and electronic equipment (WEEE) as unsorted municipal waste and to collect these WEEE separately. The collection of these wastes (WEEE) will be carried out through the public collection service of WEEE, directly and free of charge by SC Total Technologies® SRL and through collection centers organized by economic operators authorized to collect WEEE. Customers can deliver WEEE for free at the collection points specified at the time of purchase of a new product in the same category.
Thus, SC Total Technologies® SRL applies the policy of collecting WEEE in the system of taking over the equipment one by one, free of charge, according to the legislation in force, if the delivered equipment is equivalent and has fulfilled the same functions as the newly supplied equipment. Total Technologies® SRL clients can deliver equivalent WEEE at the company headquarters, during the working hours or they can announce the wish to hand over a WEEE of the same type bought on www.smartscan.ro in the order form.
Disputes and final dispositions
Any other aspect that is not already presented in any article of this document will be resolved amicably within 30 working days from the date of written notification of the problems, by the user. In case the conflict has not been resolved amicably, the competence rests with the Romanian courts, Total Technologies® SRL choosing the jurisdiction of the courts of the Bucharest municipality.
Once the agreement regarding this Agreement with Total Technologies® SRL is expressed, the client assumes all these risks.
Request Offer
Terms and conditions
The company that manages this website (www.smartscan.ro), is SC Total Technologies® SRL, registered with the Trade Register with no. J40 / 15732/1992 and having the Unique Registration Code RO1590813.
Before using the SmartScan online store, we recommend that you read the Terms and Conditions below carefully to make sure you agree with them.
Total Technologies® SRL has the right to change these provisions without prior notification, so we recommend you to check the latest version of the "Terms and Conditions" page in the "Legal" section, which is in the header of the website pages.
Copyright
The entire content of this website: images, texts, graphs, symbols, web graphics elements, scripts, programs and other data is the property of Total Technologies® SRL and its suppliers and is protected by the Copyright Law and the laws on intellectual and industrial property. . The use without the written consent of Total Technologies® SRL of any of the elements listed above is punishable according to the laws in force.
Access to information
Users of the Total Technologies® SRL platform have limited access and only in their personal interest on this website and have no right to download, modify, reproduce, copy, sell / resell or exploit the content in whole or in part, in any other manner, for commercial purposes or contrary to the interests of Total Technologies® SRL, without its prior written consent.
Limitation of liability for products and services
The products and information we offer are transmitted in exactly the same form by our manufacturers, being available within the limit of the stock. Total Technologies® SRL does not offer any warranty, expressly or implicitly, regarding the information, content, materials or products existing in the online store, as well as their suitability for a particular purpose.
Users expressly agree that the use of this online store and the purchase of products or services is at their own risk.
Also, Total Technologies® SRL cannot guarantee the availability in stock of all the presented products and does not assume the responsibility for the incorrect display of the information regarding their existence in stock. The images published on this website are for example, and the products delivered can sometimes differ from the images presented, in any way (color, accessories, appearance, etc.).
Total Technologies® SRL and its suppliers reserve the right to modify the technical specifications of the products without any prior notification. The content of the online store (texts, product descriptions, technical characteristics, images, symbols) is made in close collaboration with the representatives of the respective brands. For this reason, Total Technologies® SRL assumes no responsibility for the inappropriate descriptions of the products presented on this website, these being identical to those made available by the representatives of each brand.
All products marketed through this website are accompanied by the original warranty certificate, issued by the importer or supplier.
The maximum value of the damages that can be paid by Total Technologies® SRL to any client in the case of improper delivery or delivery is the amount of the amount collected Total Technologies® SRL from the respective client.
Regardless of how the order is transmitted (through the online store, by e-mail, telephone or other method), the acceptance of the order, ie the conclusion of the will agreement and the purchase sale contract is made when the customer signs the accompanying tax invoice the product or delivery documents made available by the fast courier company. The tax invoice takes place in the Sale-Purchase Contract. The launch of the order, the automatic email that is sent after receiving the order, the telephone discussions, by email or other methods do not constitute firm acceptance of the order and, as a result, does not mean the conclusion of the distance contract.
Limitation of liability for prices
Total Technologies® SRL reserves the right to modify without first announcing any of the prices of the products, as well as to eliminate some products, to replace them or to introduce new ones. The prices presented include VAT (19%), but not the delivery costs, unless otherwise specifically stated on the online store. The purchase price printed on the invoice will be the same as the one published on the online store at the time of purchase. There is no minimum order price limit.
Limitation of liability for delivery
Total Technologies® SRL reserves the right to contact the clients in order to confirm the orders, before their honor.
The products are delivered anywhere in Romania, within 7 (seven) working days from the moment of confirmation of the order by the TOTAL TECHNOLOGIES SRL staff.
In case the customer is not found at the address mentioned in the order, within the hourly interval agreed with the representative of the courier company, he will return again. If not this time can not make the delivery, the order will be canceled and the product returned to the premises, the customer will bear the costs of a new delivery, regardless of the value of the ordered products.
For orders that exceed the value of 150 lei (without VAT), the delivery of the products is free in the area of coverage of the courier companies. If the locality in which the delivery is to be made is not in the area of coverage of the courier companies, the client will be informed about the transport tax to be paid.
If the value of the order is less than the sum of 150 lei (excluding VAT), the delivery fee falls to the Beneficiary (Buyer).
Total Technologies® SRL reserves the right to delay or cancel deliveries of ordered products if they cannot be honored for independent reasons, such as: fires, explosions, floods, epidemics, strikes, government actions, wars, acts of terrorism, protests, riots, civil unrest or other force majeure impediments, according to Romanian law users.
Limitation of liability for payment
The payment of the ordered products can be made online with bank cards; reimbursement, to the representative of the courier company at the moment of receiving the product or by cash / bank card, when picking up the products from the Total Technologies® SRL showrooms.
Total Technologies® SRL reserves the right to request payment in advance in certain cases, based on a proforma invoice issued and transmitted through the means of communication established in agreement with the client.
Limitation of liability for product warranty
The products purchased through the online store benefit from the warranty provided by the supplier of each brand, in compliance with the legal provisions in force. Thus, each product will be accompanied by the guarantee certificate issued by the manufacturer or importer, which provides, directly or through its representatives, service for that product.
Limitation of liability for user comments
Users who visit Total Technologies® SRL's website may comment on its website and the products it offers, which may be published after the prior approval of the administrator. Total Technologies® SRL reserves the right not to publish comments with illegal, obscene, threatening, defamatory content, which in any way disturbs the private life of other persons, infringes the intellectual property rights, contain viruses, texts regarding various promotional campaigns, letters in chain, mass emails or any other form of spam. Total Technologies® SRL will not be liable nor will it be obliged to pay damages for any form of damage caused by such comments or communications.
In the case of sending materials / documents, other than those related to the process of buying / selling some products, it is considered that the user confers Total Technologies® SRL and its affiliates / associates the non-exclusive, unlimited, free, irrevocable and retransmissible right to use them, reproduce, modify, adapt, publish, translate, as well as the right to distribute them anywhere in the world, by any means.
The user guarantees that he has all the rights to the content he transmits to be published on the website, by any means, so that, by using this content, he does not cause any harm to any person. Total Technologies® SRL assumes no responsibility for the possible damages caused by the use of this information, being offered under the same limitation of liability as the rest of the content.
Limitation of liability for links to other online stores, server, browser
The website www.smartscan.ro is hosted on the servers of a third company. Total Technologies® SRL will not be held liable for any errors that may occur, regardless of the reasons for their occurrence, including changes to the website, settings or updates. Also, Total Technologies® SRL will not be liable for errors arising due to the use of certain navigation programs (browser).
Total Technologies® SRL is not responsible for the content, quality or nature of the Internet pages that can be accessed through links on the smartscan.ro website. For the respective pages, the integral responsibility is borne by their owners.
The right to return the products
The customer has the right to notify in writing Total Technologies® SRL that he gives up the purchase, without penalties and without invoking a specific reason, within 14 working days of receiving the product.
Within this term, the product purchased in the original packaging, with all the accessories and without showing any trace of damage or wear, will be returned, the customer bearing the shipping costs according to the Romanian laws.
This right can not be invoked for products picked up from our premises, for which the sample was tested or installed.
If the product is returned in a state that no longer allows the sale of the product as new, Total Technologies® SRL reserves the right to request compensation for its return to the initial stage (if possible) or to cover the price difference resulting from the sale. of the product as used. If none of these options is accepted by the customer, the product will be shipped back to him at his expense. This clause applies according to O.G. 34/2014, in the case of the purchase of products using the techniques of remote communication, applying the definitions contained in O.G. 34/2014 art. 2 paragraph 7.
Any such request / notification must be sent in writing to totaltech[at]totaltech.ro.
Protection of personal data
Total Technologies® SRL respects the private character and security of the processing of personal data of each person who visits or orders products on this site being registered under Nr. 27939 to the National Supervisory Authority for the Processing of Personal Data. - check if it is also valid for Total Technologies® !!!
According to the requirements of Law no. 677/2001 for the protection of persons regarding the processing of personal data and the free movement of these data, modified and completed, and of Law no. 506/2004 regarding the processing of personal data and the protection of privacy in the electronic communications sector, Total Technologies® SRL will manage, in safe conditions and only for the specified purposes, the personal data provided.
It is obligatory to provide the data, these being necessary to be able to be contacted, to be able to issue the transaction documents and to deliver the order. Refusal to provide this information leads to the impossibility of processing the placed orders.
The registered information is intended for use by Total Technologies® SRL and is communicated (partially) only to the following recipients: the fast courier company that delivers the products, the company that provides the service, in the case of the products that require installation / interventions within the guarantee term and the banking institutions or financial, for card payments (online or directly in the showroom).
If you would like to receive information about the products or services offered by Total Technologies® SRL, please check the subscription box on the newsletter at the end of an order or subscribe directly through the dedicated section of the website. In case you wish to subsequently receive communications from Total Technologies® SRL, please unsubscribe using the link in each newsletter.
According to the Law no. 677/2001 you benefit from the right of access and intervention on the data, as well as the right not to be subjected to an individual decision. At the same time, you have the right to oppose the processing of personal data concerning you and to request their deletion. In order to exercise these rights, you can address a written request sent by email (totaltech[at]totaltech.ro), dated and signed on the address of Total Technologies® SRL, which can be found on the contact page. However, it should be specified that, in accordance with the provisions of the legislation in force, the personal data cannot be erased from the financial, fiscal and management documents issued by Total Technologies® SRL, these processing having a mandatory character, and Law no. 677/2001 provides for the exclusion of the right of opposition in this case. If some of the data provided is incorrect, please inform us as soon as possible at totaltech[at]totaltech.ro.
Security of personal data
Your personal data will be used by Total Technologies® SRL and its suppliers only for the stated purpose of this website, in accordance with the legal provisions.
The information in the order form will be used to send the order confirmation, any promotions, periodic newsletters, etc. Total Technologies® SRL undertakes not to make public or sell databases containing information regarding personal data.
The personal data may be transmitted to the authorities in the right to verify the commercial transactions, only at their justified request.
Information security
The security level of transactions, purchasing sessions and the introduction of personal data is in accordance with the requirements of this industry. This website uses security measures against the loss, alteration or misuse of the information that is under our control by securing the platform with Cross-Site Scripting. However, Total Technologies® SRL assumes no responsibility for the loss of information caused by bugs, "hacking" attacks or errors in the software with which the online store is designed and hosted.
Promotions and contests
Total Technologies® SRL establishes the regulations of the promotions and competitions that it organizes and informs the possible participants through its own online store or through e-mail messages. Promotions benefit only those orders that comply precisely with the rules posted on the site. Also, promotions apply only to orders that are registered during their validity and only within the limit of the available stock.
Total Technologies® SRL does not guarantee the availability on stock of the products for promotion and may interrupt or cancel at any time without any prior notification.
Waste electrical and electronic equipment (WEE)
Waste electrical and electronic equipment (WEEE) may contain hazardous substances that have a negative impact on the environment and human health if not collected separately.
Considering the provisions of GEO 195/2005, regarding the protection of the environment and O.U.G. 5/2015 regarding the waste of electrical and electronic equipment, the buyers have the obligation not to eliminate the waste of electrical and electronic equipment (WEEE) as unsorted municipal waste and to collect these WEEE separately. The collection of these wastes (WEEE) will be carried out through the public collection service of WEEE, directly and free of charge by SC Total Technologies® SRL and through collection centers organized by economic operators authorized to collect WEEE. Customers can deliver WEEE for free at the collection points specified at the time of purchase of a new product in the same category.
Thus, SC Total Technologies® SRL applies the policy of collecting WEEE in the system of taking over the equipment one by one, free of charge, according to the legislation in force, if the delivered equipment is equivalent and has fulfilled the same functions as the newly supplied equipment. Total Technologies® SRL clients can deliver equivalent WEEE at the company headquarters, during the working hours or they can announce the wish to hand over a WEEE of the same type bought on www.smartscan.ro in the order form.
Disputes and final dispositions
Any other aspect that is not already presented in any article of this document will be resolved amicably within 30 working days from the date of written notification of the problems, by the user. In case the conflict has not been resolved amicably, the competence rests with the Romanian courts, Total Technologies® SRL choosing the jurisdiction of the courts of the Bucharest municipality.
Once the agreement regarding this Agreement with Total Technologies® SRL is expressed, the client assumes all these risks.




